Research homepage Sebastian Mueller, MD, PhD
OLD WEB PAGE till 2016
Liver stiffness - a novel parameter for the diagnosis of liver diseases
A better understanding of liver disease and the development of novel diagnostic tools and therapeutic approaches is in the center of our basic research and translational research activities. Below are some recent data from our group on the recently introduced novel parameter of liver stiffness which has drastically improved the diagnosis of chronic liver disease. Liver stiffness has also given novel inspirations for a better molecular understanding of liver cirrhosis, the major end-stage disease of all liver diseases that still cannot be treated causally and whose pathophysiology is still poorly understood.
Selected topics
1. Improved algorithms for fibrosis assessment using liver stiffness
2. Molecular mechanisms of liver stiffness
3. The sinusoidal pressure hypothesis to explain liver cirrhosis and the role of arterialization
4. Genetics of liver cirrhosis and alcoholic liver disease
1. Diagnosis and Improved algorithms for fibrosis assessment using liver stiffness
The noninvasive quantitation of liver stiffness (LS) by ultrasound based transient elastography using FibroScan® has revolutionized the diagnosis of liver diseases, namely liver cirrhosis (reviewed in ref. 41). Alternative techniques such as acoustic radiation impulse frequency imagingsupersonic imaging or magnetic resonance elastography are currently under investigation. LS is an excellent surrogate marker of advanced fibrosis (F3) and cirrhosis (F4) outscoring all previous noninvasive approaches to detect cirrhosis. LS values below 6 kPa are considered as normal and exclude ongoing liver disease. LS of 8 and 12.5 kPa represent generally accepted cut-off values for F3 and F4 fibrosis. LS highly correlates with portal pressure, and esophageal varices are likely at values >20 kPa. Many other factors may also increase LS.
We have proposed that prior to liver stiffness interpretation
a) LS-modulating factors such as congestion, cholestasis, other spatial irregularities should be excluded by ultrasound
b) inflammation and hepatic damage should be assessed by transaminase levels
c) an intervention (e.g. alcohol detoxification, treatment of congestive heart failure with diuretics etc.) should be considered to remove the LS-increasing effect of such factors.
The final liver stiffness much better reflects the true fibrosis stage. Our actual practiced algorithm is shown below.
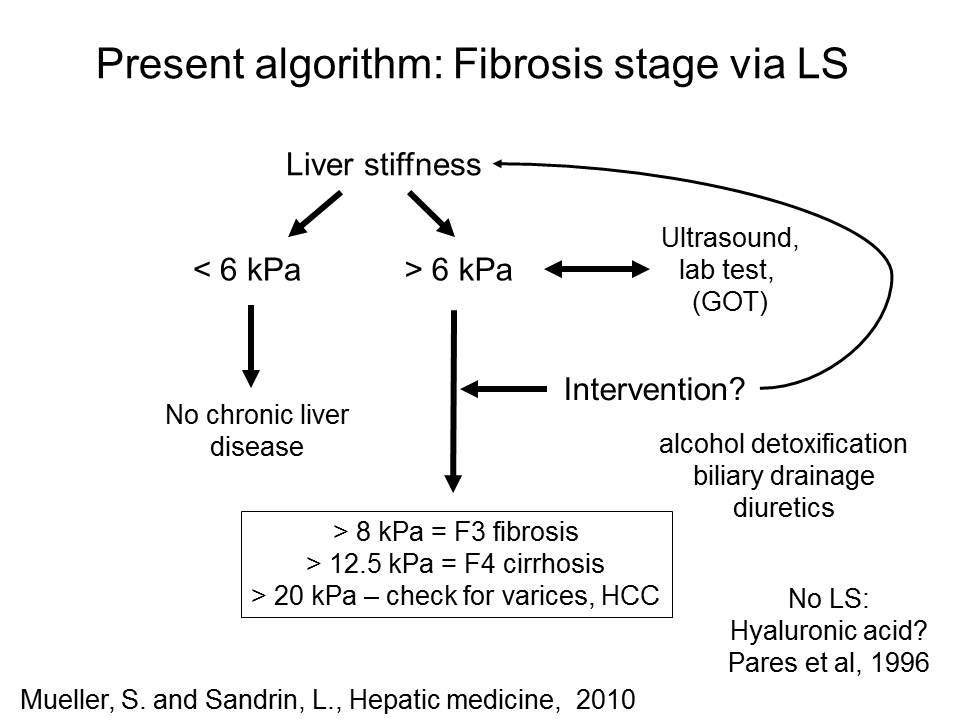
Fig. 1: Liver stiffness interpretation using ultraound, transaminase levels and potential interventions
2. Molecular mechanisms of liver stiffness (LS), concepts since 2008
Our group has extensively studied factors other than fibrosis that affect liver stiffness (see Fig. 2 and 3 below). Thus, we were able to show that liver congestion and cholestasis can drastically increase liver stiffness irrespective of the fibrosis degree. Thus, LS should always be interpreted in the context of clinical, imaging and laboratory findings. In addition, we could recently show that liver stiffness can also be determined in the presence of ascites using transient elastography. This allows rapid identification of patients with non-hepatic causes of ascites. Our recent data also suggest that liver stiffness much better reflects liver function as thought so far.


Fig. 2 Liver stiffness caused by matrix and pressure Fig. 3: Liver stiffness range caused by various factors.
3. Novel sinusoidal pressure hypothesis (SPH) to explain liver cirrhosis (established in 2010)
Together with L. Sandrin, we had developed in 2010 the then called pressure-stiffness-matrix hypothesis to explain liver cirrhosis (presented in ref. 41). Fig. 4 and 5 below schematically show the new principle in comparison to the conventional view. We believe that all liver diseases whether of inflammatory- or non-inflammatory origin lead to increased pressure. This pressure causes stretching of the persinusoidal space which is the actual signal for matrix production e.g. by hepatic stellate cells.We postulate further that the secreted matrix corresponds to the pressure in terms of time and intensity. Liver stiffness is ultimately the results of increased pressure or/and matrix deposition.


Fig. 4 and 5: Pressure-Matrix hypothesis of liver fibrosis
4. Extended sinusoidal pressure hypothesis (SPH) (updated in 2016)
Meanwhile, the pressure hypothesis has been significantly matured and it is now called sinusoidal pressure hypothesis. Updates have been presented and discussed at various meetings including in Aachen and at the EASL monothematic concerence on liver fibrosis held in Porto in 2016.
A
detailed updated manuscript on SPH is in preparation. Fig. 6 below summarized
important novel findings on the role of matrix and pressure on liver stiffness.
Fig. 7A shows the updated concept of SPH. Part I (initiation) encompasses
elevation of sinusodial pressure with stretch-force induced mechanosignaling
ultrimately leading to matrix deposition (fibrosis). The elevated stiffness and
vascular resistance finally lead to a predominant arterial blood supply
(arterialization) which exposes the liver permanently to high pressures (SPH
Part II, perpetuation). Thus, vicious cycle is initiated that ultimately causes
irreversible liver cirrhosis. The
SPH is able to explain the macroscopic changes of the cirrhotic liver and the
uniform fibrotic response to various etiologies. This novel concept will
hopefully stimulate the search for new treatment strategies. Fig. 7B (below) highlights
the concept of SPH at the cellular level. Here,
sinusoidal pressure
is
the actual driving force for the production of collagen by stretching of
perisinusoidal cells, pressure-related increase in tissue stiffness and stretch
forces transduced via cellular and intercellular biomechanic signaling.
Fig. 6: Both matrix and pressure affect liver stiffness. Fig. 7: Sinusodial pressure hypothesis at the A) tissue and B) cellular level (see text above).
1. Futher optimization of diagnostic algorithms for chronic liver diseases using liver stiffness.
2. How to determine fibrosis scores in the presence of hepatitis?
2. Better definition of the indications and limitations of the various technologies to determine liver stiffness (e.g. transient Elastography (Fibroscan), ARFI, Supersonic Imaging, Magnetic Resonance Elastography)
3. Identification of additional factors affecting liver stiffness.
4. Validation of sinusoidal pressure hypothesis.
References Liver stiffness (Mueller group from Heidelberg)
79. Arterial pressure suffices to increase liver stiffness.
Pecha F, Peccerella T, Bruckner T, Seitz HK, Rausch V, Mueller S. Am J Physiol Gastrointest Liver Physiol. 2016 Jun 10:ajpgi.00399.2015. doi: 10.1152/ajpgi.00399.2015. [Epub ahead of print]
PMID:27288426 Similar articles
Mueller S, Englert S, Seitz HK, Badea RI, Erhardt A, Bozaari B, Beaugrand M, Lupșor-Platon M. Liver Int. 2015 Jun 30. doi: 10.1111/liv.12904. [Epub ahead of print]
63. Direct comparison of the FibroScan XL and M probes for assessment of liver fibrosis in obese and nonobese patients Durango E, Dietrich C, Seitz HK, Kunz CU, Pomier-Layrargues GT, Duarte-Rojo A, Beaton M, Elkhashab M, Myers RP, Mueller S Hepatic Medicine: Evidence and Research Published Date July 2013 Volume 2013:5 Pages 43 – 52 DOI: http://dx.doi.org/10.2147/HMER.S45234
59.
Assessment
of renal allograft fibrosis by transient elastography.
Sommerer C, Scharf M, Seitz C, Millonig G, Seitz HK, Zeier M, Mueller S. Transpl
Int. 2013 May;26(5):545-51. doi: 10.1111/tri.12073. Epub 2013 Feb 6. PMID:
23383606 [PubMed - in process] Related
citations
58.
Noninvasive
assessment of patients with alcoholic liver disease Mueller,
S. (2013), Clinical
Liver Disease, 2: 68–71. doi: 10.1002/cld.186Article
first published online: 24 APR 2013 DOI: 10.1002/cld.186
56.
Systemic mastocytosis – a rare case of increased liver stiffness
Stefanie Adolf, Gunda Millonig, H. K. Seitz, Andreas Reiter, Peter Schirmacher, Thomas Longerich, and Sebastian Mueller
Case Reports in Hepatology, vol. 2012, Article ID 728172, 6 pages,
2012. doi:10.1155/2012/728172 Received 2 September 2012; Accepted 19 September 2012
54.
Association
of Liver Stiffness with Hepatic Expression of Pharmacokinetically
Important Genes in Alcoholic Liver Disease.
Theile
D, Haefeli WE, Seitz HK, Millonig G, Weiss J, Mueller S.
Alcohol
Clin Exp Res. 2012 Jul 24. doi: 10.1111/j.1530-0277.2012.01901.x. [Epub
ahead of print]
PMID:
22827451 [PubMed - as supplied by publisher]
52. EASL
Clinical Practical Guidelines: Management of Alcoholic Liver
Disease.
European Association For The Study Of The
Liver.
J
Hepatol. 2012 May 25. [Epub ahead of print] No
abstract available.
PMID: 22633836
[PubMed - as supplied by publisher]
51. Transient
elastography with the XL probe rapidly identifies patients with
non-hepatic ascites.
Kohlhaas, A.; Durango, E.; Millonig, G.; Bastard,
C.; Sandrin, L.; Golriz, M.; Mehrabi, A.; Büchler, M. W.; Seitz, H. K.;
Mueller, S. Hepatic
Medicine: Evidence and Research 4:11-18; 2012.
47. Transient
micro-elastography: A novel non-invasive approach to measure liver
stiffness in mice.
Bastard
C, Bosisio MR, Chabert M, Kalopissis AD, Mahrouf-Yorgov M, Gilgenkrantz H,
Mueller S, Sandrin L.
World
J Gastroenterol. 2011 Feb
28;17(8):968-75. Free PMC Article Related
citations
41. Liver
stiffness: a novel parameter for the diagnosis of liver disease
Sebastian
Mueller, Laurent Sandrin
Hepatic
Medicine: Evidence and Research
Published Date May 2010 , Volume 2010:2 Pages 49 -
67
38. Increased
liver stiffness in alcoholic liver disease: Differentiating fibrosis from
steatohepatitis.
Mueller
S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, Eisele S,
Stickel F, Longerich T, Schirmacher P, Seitz HK.
World
J Gastroenterol.
2010 Feb 28;16(8):966-72.PMID:
20180235 [PubMed - in process] Related
articlesFree
article
35. Liver
stiffness is directly influenced by central venous pressure.
Millonig
G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl
G, Büchler MW, Seitz HK, Mueller S.
J
Hepatol. 2009
Dec 4. [Epub ahead of print]PMID:
20022130 [PubMed - as supplied by publisher] Related
articles
26. Extrahepatic
cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis.
Hepatology. 2008 Nov;48(5):1718-23. PMID: 18836992 [PubMed - indexed for MEDLINE] Related Articles