Research homepage Sebastian Mueller, MD, PhD
Liver stiffness: The novel non-invasive gold standard to screen for liver diseases and a new approach to understand liver cirrhosis
A better understanding of liver disease and the development of novel diagnostic tools and therapeutic approaches is in the center of our basic research and translational research activities. Below are some recent data from our group on the recently introduced novel parameter of liver stiffness which has drastically improved the diagnosis of chronic liver disease. Liver stiffness has also given novel inspirations (Sinusoidal pressure hypothesis)for a better molecular understanding of liver cirrhosis, the major end-stage disease of all liver diseases that still cannot be treated causally and whose pathophysiology is still poorly understood.
Selected topics
1. Improved algorithms for fibrosis assessment using liver stiffness
2. The sinusoidal pressure hypothesis to explain liver cirrhosis and the role of arterialization
1. Diagnosis and Improved algorithms for fibrosis assessment using liver stiffness
The noninvasive quantitation of liver stiffness (LS) by ultrasound based transient elastography using FibroScan® has revolutionized the diagnosis of liver diseases, namely liver cirrhosis (reviewed in ref. 41). Alternative techniques such as acoustic radiation impulse frequency imagingsupersonic imaging or magnetic resonance elastography are currently under investigation. LS is an excellent surrogate marker of advanced fibrosis (F3) and cirrhosis (F4) outscoring all previous noninvasive approaches to detect cirrhosis. LS values below 6 kPa are considered as normal and exclude ongoing liver disease. LS of 8 and 12.5 kPa represent generally accepted cut-off values for F3 and F4 fibrosis. LS highly correlates with portal pressure, and esophageal varices are likely at values >20 kPa. Many other factors may also increase LS.
We have proposed that prior to liver stiffness interpretation
a) LS-modulating factors such as congestion, cholestasis, other spatial irregularities should be excluded by ultrasound
b) inflammation and hepatic damage should be assessed by transaminase levels
c) an intervention (e.g. alcohol detoxification, treatment of congestive heart failure with diuretics etc.) should be considered to remove the LS-increasing effect of such factors.
The final liver stiffness much better reflects the true fibrosis stage. Our actual practiced algorithm is shown below.
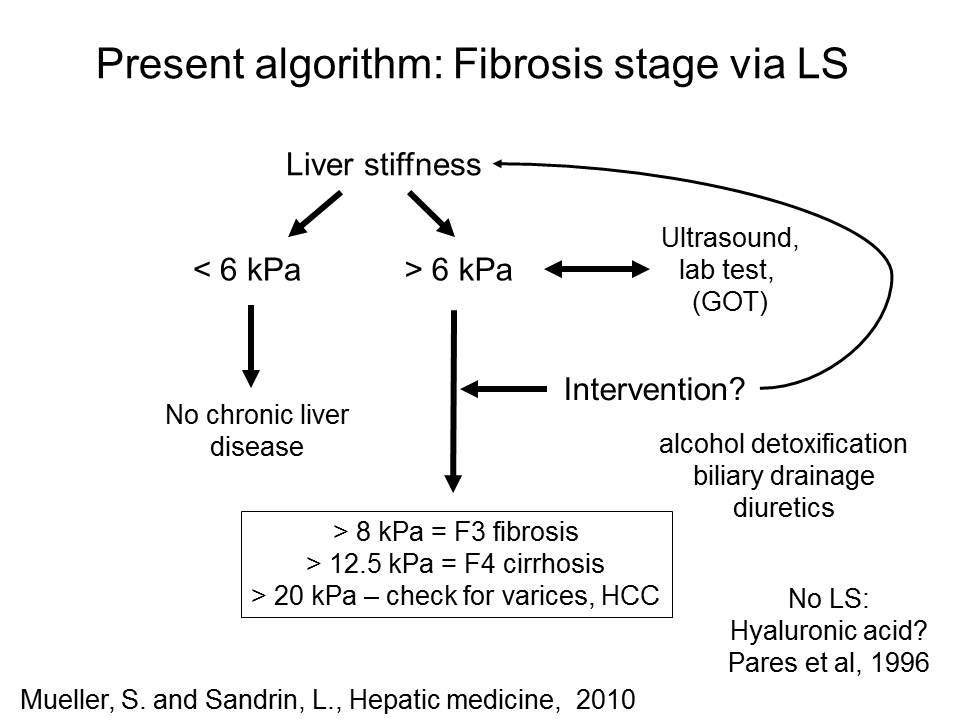
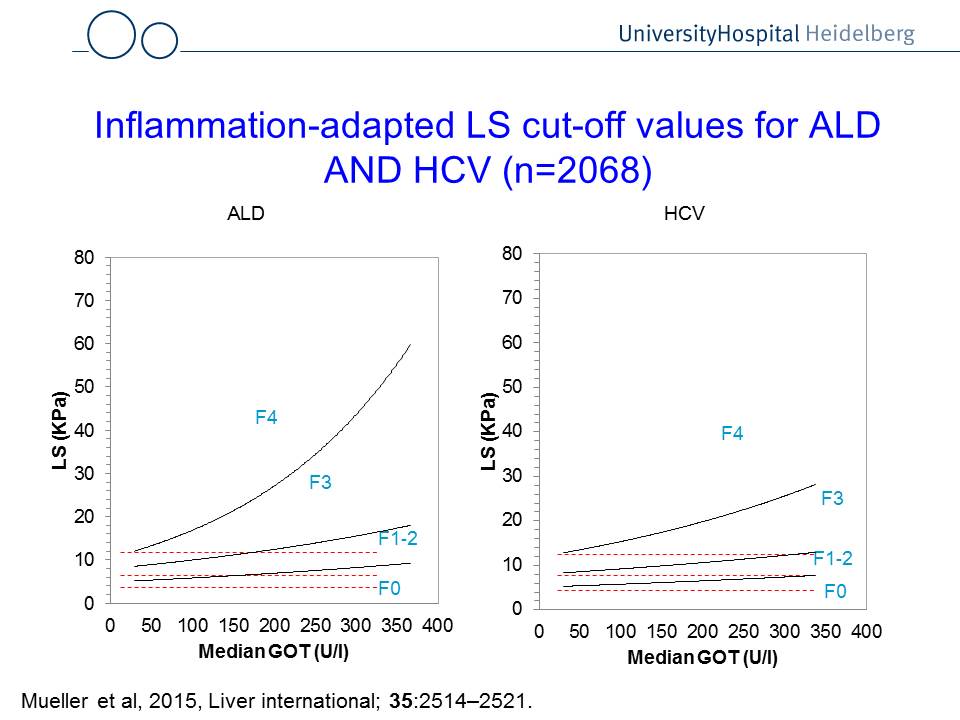
Fig. 1: Liver stiffness interpretation using ultraound, transaminase levels and potential interventions (left panel). The right panel shows GOT (AST)-adapted cut-off values to assess liver fibrosis. For instance, if a patient with hepatitis C (HCV) has a GOT of 200 U/l, the cut-off value for F4 (cirrhosis) is ca. 20 kPa. If the a patient suffers from alcoholic liver disease (ALD), the same GOT levels result in a cut-off values for F4 cirrhosis at ca. 30 kPa.
3. Sinusoidal pressure hypothesis (SPH) (updated in 2016)
In
2016, my paper
in WJG introduced the sinusoidal pressure hypothesis (SPH), which identifies
an elevated sinusoidal pressure (SP) as cause of fibrosis. SPH has been mainly
derived from recent studies on liver stiffness (see also references below). Fig.
2 below summarizes the present concept on the role of matrix and pressure on liver stiffness.
Fig. 3 demonstrates the principle of the sinusoidal pressure hypothesis (SPH). Part I (initiation) encompasses
elevation of sinusodial pressure with stretch-force induced mechanosignaling
ultrimately leading to matrix deposition (fibrosis). The elevated stiffness and
vascular resistance finally lead to a predominant arterial blood supply
(arterialization) which exposes the liver permanently to high pressures (SPH
Part II, perpetuation). Thus, vicious cycle is initiated that ultimately causes
irreversible liver cirrhosis. The
SPH is able to explain the macroscopic changes of the cirrhotic liver and the
uniform fibrotic response to various etiologies. This novel concept will
hopefully stimulate the search for new treatment strategies. Fig. 4 (below) highlights
the concept of SPH at the cellular level. Here,
sinusoidal pressure
is
the actual driving force for the production of collagen by stretching of
perisinusoidal cells, pressure-related increase in tissue stiffness and stretch
forces transduced via cellular and intercellular biomechanic signaling.
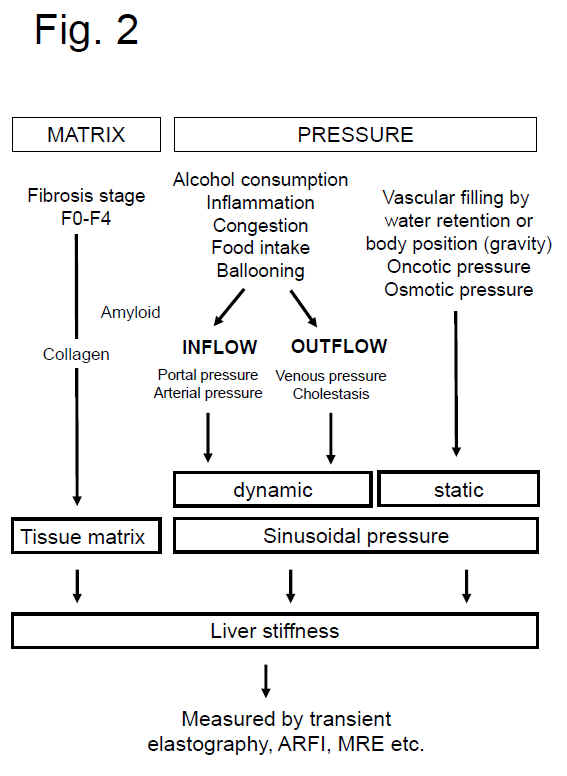
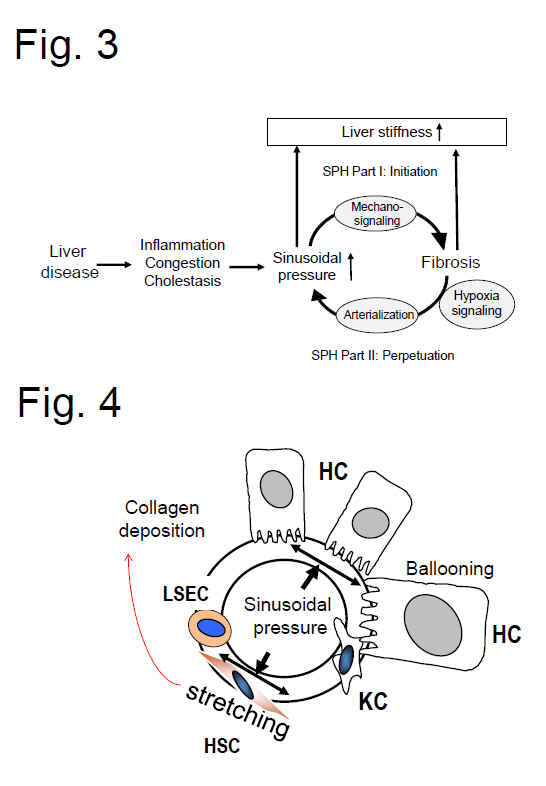
Fig. 2: Both matrix and pressure affect liver stiffness. Sinusodial pressure hypothesis at the tissue (Fig. 3) and cellular level (Fig. 4) (see text above). According to SPH, SP predominantly translates into mechanic stretch forces within the perisinusoidal bed. Hepatocyte cell death, inflammation or congestion all lead to increased SP that causes stretching of e.g., hepatic stellate cells (HSC), liver sinus endothelial cells (LSEC) or hepatocytes (HC).
SPH at the hemodynamic level: Sinusoidal pressure as consequence of the hepatic inflow/outflow balance and static/dynamic components
Fig. 6 shows a simplified scheme of the vascular and biliary architecture of the liver to better illustrate the role of the various inflow, outflow and shunt factors on SP. Based on the physics of liquids, SP will be mainly determined by static and dynamic components (see also Fig. 3). The static part of the SP is determined by the intravasal pressure and the elastic properties of the vessels walls and also exists in the absence of a functioning blood circulation. Osmotic, oncotic pressure as well as gravitational forces related to the body positioning further contribute to this component. In contrast, the dynamic component is represented by the kinetic energy of the blood flow and becomes only relevant under conditions of an operating blood circulation. Here, the flow resistance constituted by the liver and the blood flow rate generated by the heart will both affect SP. Moreover, the dynamic part of SP will depend on the localization of e.g. the inflammation. Inflammatory components such as cellular swelling or infiltration of inflammatory cells will all increase the vascular resistance locally either in the portal or central areas.
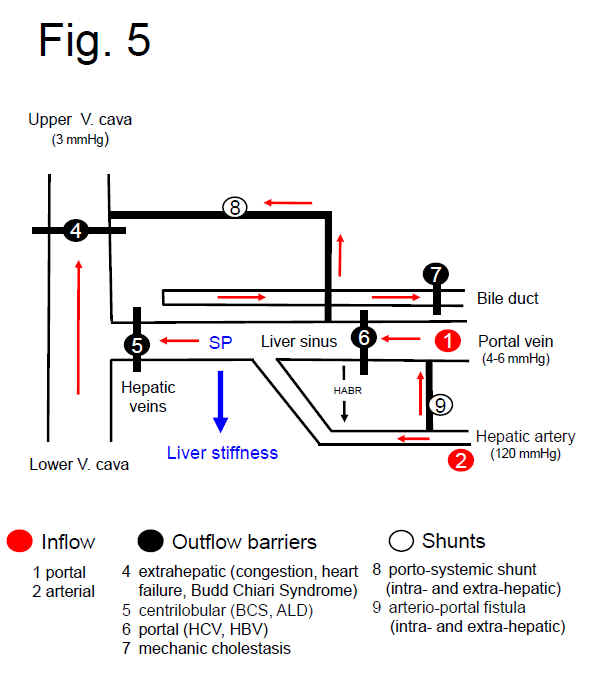
Fig. 5. SPH at the hemodynamic, vascular level. Simplified scheme of the hepatic vascular architecture and conditions that result in elevated SP (SP) and liver stiffness (LS). SP and LS are shown as consequence of the various inflow (red circles) and outflow balances (black circles) in a schematized vascular architecture of the liver. Intrahepatic or extrahepatic shunts (white circles) will also affect SP in a complex manner and, according to SPH, will also have an important impact on the development of portal hypertension and liver function. According to SPH, M1 (stiff livers with good liver function) and M2 (soft liver with poor liver function) can be postulated for cirrhotics based on intrahepatic shunt formation. Red arrows: flow direction, HABR: hepatic arterial buffer response
1. Futher optimization of diagnostic algorithms for chronic liver diseases using liver stiffness.
2. How to determine fibrosis scores in the presence of hepatitis?
2. Better definition of the indications and limitations of the various technologies to determine liver stiffness (e.g. transient Elastography (Fibroscan), ARFI, Supersonic Imaging, Magnetic Resonance Elastography)
3. Identification of additional factors affecting liver stiffness.
4. Validation of sinusoidal pressure hypothesis.
References Liver stiffness (S. Mueller, CAR, Heidelberg)
for details see also book on liver stiffness published in 2020::
S. Mueller, Liver Elastography: Clinical Use and Interpretation in English (Springer Nature, 2020).
83.
Does
pressure cause liver cirrhosis? The sinusoidal pressure hypothesis.
Mueller
S.
World
J Gastroenterol 2016; 22(48):
10482-10501
82. Primary liver injury and delayed resolution of liver stiffness after alcohol detoxification in heavy drinkers with the PNPLA3 variant I148M.
Rausch V, Peccerella T, Lackner C, Yagmur E, Seitz HK, Longerich T, Mueller S.
World J Hepatol. 2016 Dec 18;8(35):1547-1556. doi: 10.4254/wjh.v8.i35.1547.
PMID: 28050235
79. Arterial pressure suffices to increase liver stiffness.
Pecha F, Peccerella T, Bruckner T, Seitz HK, Rausch V, Mueller S. Am J Physiol Gastrointest Liver Physiol. 2016 Jun 10:ajpgi.00399.2015. doi: 10.1152/ajpgi.00399.2015. [Epub ahead of print]
PMID:27288426 Similar articles
Mueller S, Englert S, Seitz HK, Badea RI, Erhardt A, Bozaari B, Beaugrand M, Lupșor-Platon M. Liver Int. 2015 Jun 30. doi: 10.1111/liv.12904. [Epub ahead of print]
63. Direct comparison of the FibroScan XL and M probes for assessment of liver fibrosis in obese and nonobese patients Durango E, Dietrich C, Seitz HK, Kunz CU, Pomier-Layrargues GT, Duarte-Rojo A, Beaton M, Elkhashab M, Myers RP, Mueller S Hepatic Medicine: Evidence and Research Published Date July 2013 Volume 2013:5 Pages 43 – 52 DOI: http://dx.doi.org/10.2147/HMER.S45234
59. Assessment
of renal allograft fibrosis by transient elastography.
Sommerer C, Scharf M, Seitz C, Millonig G, Seitz HK, Zeier M, Mueller S. Transpl
Int. 2013 May;26(5):545-51. doi: 10.1111/tri.12073. Epub 2013 Feb 6. PMID:
23383606 [PubMed - in process] Related
citations
58. Noninvasive
assessment of patients with alcoholic liver disease Mueller,
S. (2013), Clinical
Liver Disease, 2: 68–71. doi: 10.1002/cld.186Article
first published online: 24 APR 2013 DOI: 10.1002/cld.186
56. Systemic mastocytosis – a rare case of increased liver stiffness
Stefanie Adolf, Gunda Millonig, H. K. Seitz, Andreas Reiter, Peter Schirmacher, Thomas Longerich, and Sebastian Mueller
Case Reports in Hepatology, vol. 2012, Article ID 728172, 6 pages,
2012. doi:10.1155/2012/728172 Received 2 September 2012; Accepted 19 September 2012
54. Association
of Liver Stiffness with Hepatic Expression of Pharmacokinetically
Important Genes in Alcoholic Liver Disease.
Theile
D, Haefeli WE, Seitz HK, Millonig G, Weiss J, Mueller S.
Alcohol
Clin Exp Res. 2012 Jul 24. doi: 10.1111/j.1530-0277.2012.01901.x. [Epub
ahead of print]
PMID:
22827451 [PubMed - as supplied by publisher]
52. EASL
Clinical Practical Guidelines: Management of Alcoholic Liver
Disease.
European Association For The Study Of The
Liver.
J
Hepatol. 2012 May 25. [Epub ahead of print] No
abstract available.
PMID: 22633836
[PubMed - as supplied by publisher]
51. Transient
elastography with the XL probe rapidly identifies patients with
non-hepatic ascites.
Kohlhaas, A.; Durango, E.; Millonig, G.; Bastard,
C.; Sandrin, L.; Golriz, M.; Mehrabi, A.; Büchler, M. W.; Seitz, H. K.;
Mueller, S. Hepatic
Medicine: Evidence and Research 4:11-18; 2012.
47. Transient
micro-elastography: A novel non-invasive approach to measure liver
stiffness in mice.
Bastard
C, Bosisio MR, Chabert M, Kalopissis AD, Mahrouf-Yorgov M, Gilgenkrantz H,
Mueller S, Sandrin L.
World
J Gastroenterol. 2011 Feb
28;17(8):968-75. Free PMC Article Related
citations
41. Liver
stiffness: a novel parameter for the diagnosis of liver disease
Sebastian
Mueller, Laurent Sandrin
Hepatic
Medicine: Evidence and Research
Published Date May 2010 , Volume 2010:2 Pages 49 -
67
38. Increased
liver stiffness in alcoholic liver disease: Differentiating fibrosis from
steatohepatitis.
Mueller
S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, Eisele S,
Stickel F, Longerich T, Schirmacher P, Seitz HK.
World
J Gastroenterol.
2010 Feb 28;16(8):966-72.PMID:
20180235 [PubMed - in process] Related
articlesFree
article
35. Liver
stiffness is directly influenced by central venous pressure.
Millonig
G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl
G, Büchler MW, Seitz HK, Mueller S.
J
Hepatol. 2009
Dec 4. [Epub ahead of print]PMID:
20022130 [PubMed - as supplied by publisher] Related
articles
26. Extrahepatic
cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis.
Hepatology. 2008 Nov;48(5):1718-23. PMID: 18836992 [PubMed - indexed for MEDLINE] Related Articles