Mueller S, Englert S, Seitz HK, Badea RI, Erhardt A, Bozaari B, Beaugrand M, Lupșor-Platon M. Liver Int. 2015 Jun 30. doi: 10.1111/liv.12904.
BACKGROUND
& AIMS: It is
well known that inflammation increases liver stiffness (LS) in patients with
chronic hepatitis C (HCV) and alcoholic liver disease (ALD) independent of
fibrosis stage, but no inflammation-adapted cut-off values have been settled so
far. An early identification of rapid fibrosers, however, is essential to decide
whom to treat first with the novel, but expensive antiviral drugs.
METHODS:
LS, biopsy-proven fibrosis stages F0-F4 (METAVIR or Kleiner score) and routine
laboratory parameters were studied in 2068 patients with HCV (n=1391) and ALD
(n= 677).
RESULTS:
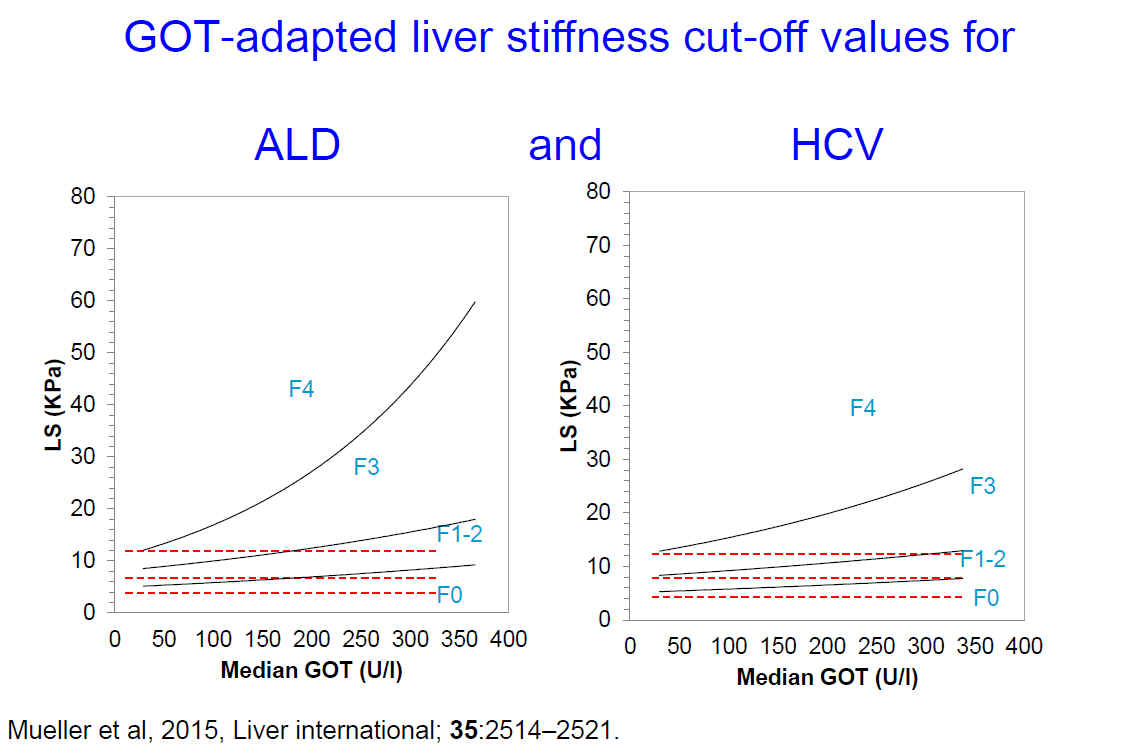
Among the routine parameters for liver damage, AST correlated best with LS (HCV:
r=0.54, p<0.0001 and ALD: r=0.34, p<0.0001). In the absence of elevated
transaminases, cut-off values were almost identical between HCV and ALD for
F1-2, F3 and F4 (HCV: 5.1, 9.0 and 11.9 kPa vs ALD: 4.9, 8.1 and 10.5 kPa).
These cut-off values increased exponentially as a function of median AST level.
The impact of AST on LS was higher in lobular-pronounced ALD as compared to
portal tract-localized HCV. Most notably, Cohenís weighted Kappa displayed an
improved agreement of the novel AST-dependent cut-off values with histological
fibrosis stage both for HCV (0.68 vs 0.65) and ALD (0.80 vs 0.76).
CONCLUSIONS:
The novel AST-adapted cut-off values improve noninvasive fibrosis staging in HCV
and ALD and may be also applied to other liver diseases. Especially in HCV, they
could help to decide whom to treat first with the novel but expensive antiviral
drugs.